Fintech has delivered on all four pillars of PM’s Digital India Vision: Rajeev Chandrasekhar
CX Partners-backed Veeda Clinical Research files for IPO to raise 831 cr
Published on September 29, 2021
New Delhi : Clinical research organisation Veeda Clinical Research Limited has filed its draft red herring prospectus (DRHP) with capital markets regulator Securities and Exchange Board of India (Sebi) on Tuesday, 28 September 2021 to raise funds from the primary markets.
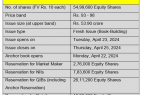
The issue will consist of an issuance of fresh equity shares worth up to Rs 331.60 crore and an offer for sale (OFS) of Rs 500 crore by promoters and existing shareholders.
Investors participating in OFS include CX Alternative Investment Fund aggregating up to Rs 8.08 crore, Rs 90.19 crore by Arabelle Financial Services Limited, Rs 259.77 crore by Bondway Investment Inc., Rs 0.04 crore by Stevey International Corporation and Rs 141.93 crore by Basil Private Limited.
The company intends to utilise net proceeds from the fresh issue for repayment of the debt, funding capital expenditure, Investing/funding further acquisition of subsidiary Bioneedsfunding working capital requirements besides general corporate purposes.
The Ahmedabad-based company is backed by CX Partners, in June announced raising $16 million from PE firms Sabre Partners, and high networth individuals including Pranab Mody of JB Chemicals, Havells India family office, Nikhil Vora of Sixth Sense Ventures and Arjun Bhartia of Jubilant Group among others, according to a report by moneycontrol. Furthermore, to support its capabilities and to offer top-notch clinical service for novel drugs, Veeda acquired a 50.1% stake in Bangalore-based Bioneeds India Private Limited, after it acquired a substantial minority stake in the company during March and July 2021.
Veeda, grown from a single facility in 2004 to now having 4 facilties and the capability to process 1L samples per month, is one of the largest independent full service clinical research organization (“CRO”) in India as of March 31, 2020, on the basis of revenue, according to the Frost & Sullivan report represented in its DRHP. It specializes in the focused segment of Bio Availability / Bio Equivalence (BA/BE) studies.In addition to BA/BE tests, it offers a broad range of services across most aspects of the drug development and drug launch value chain throughout the global markets including North America, Europe and Asia. In FY21 it has completed studies for 157 clients, some of the prominent names being Dr Reddy’s, Mankind Pharma, Granules India, Novugen Pharma.
Veeda has a full suite of clinical trials including pre-clinical, early phase and late phase clinical trials, together with related services. In particular, it states, we have expertise in pharmacokinetics (PK) studies as well as trials in generic molecules, New Chemical Entities (NCEs), large molecules and biosimilars.
As of March 2021, Veeda has conducted more than 3,500 trials and developed more than 1,000 bio-analytical methods spread across generics. It has also been able to complete over 85 global inspections with regulatory authorities like USFDA, UK-MHRA, WHO, ANVISA, DGCI and EMA.
Veeda Clinical’s revenue from operations stood at Rs 195.81 crore during the year ended March 2021, while its profit after tax during the year stood at Rs 62.97 crore.
Globally the market for biosimilars is estimated to grow at a 17.3% CAGR. India is expected to see a growth due to the large patient cliff expected in the next 5 years, more so because the reduced cost of development biosimilars in the country, less stringent approval criteria, lower labour besides other factors global generic players have begun outsourcing BA/BE trials to the country.
The CRO Market in India captures 3% of the global market share by value and is expected to be the fastest growing market with a CAGR of 12% between 2021-2026.
SBI Capital Markets Limited, ICICI Securities Limited, JM Financial Limited and Systematix Corporate Services Limited are the book running lead managers to the issue.