Syngene reports first quarter results Revenue from operations up 41% to Rs. 5,945 Mn, PAT increased 33% to Rs. 773 Mn
Published on July 21, 2021
Bangalore: Syngene International Limited today announced its first quarter results for FY22. The Company reported quarterly revenue from operations growth of 41% year-on-year to Rs. 5,945 Mn; profit after tax for the quarter increased by 33% year-on-year to Rs. 773 Mn.
Commenting on the results, Jonathan Hunt, Managing Director and Chief Executive Officer, Syngene International Limited, said, “We made a strong start to the financial year. Besides continuing progress across all our business divisions, growth for the quarter was strongly boosted by the manufacturing of COVID-19 treatment, Remdesivir, as we increased production to meet the needs of the second wave of COVID-19 in India. We also made headway with the expansion of our dedicated R&D center for Bristol Myers Squibb following the contract extension announced last quarter.
As India faced a second wave of the pandemic, our safety protocols continued to provide a sustainable work environment enabling our staff to operate at normal levels and keep client projects on track. During the quarter, following government guidance, we were proud to be able to offer vaccinations to our staff and their families as an additional level of protection.
Overall, first quarter performance was in line with our expectations and puts us on track to meet our full year growth guidance in the coming quarters.”
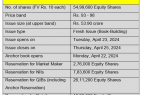
Quarterly Financial Highlights (All numbers are in Indian rupees in Million except margins)
| Q1 FY22 | Q1 FY21 | YoY Change (%) | |
| Revenue from Operations | 5,945 | 4,216 | 41 |
| Revenue | 6,068 | 4,369 | 39 |
| EBITDA | 1,773 | 1,398 | 27 |
| EBITDA margin (%) | 29 | 32 | |
| PAT | 773 | 580 | 33 |
| PAT Margin (%) | 13 | 13 |
Business updates
Syngene’s first quarter performance reflects growth across all its business divisions as the Company continued to operate at normal levels. The growth in the Dedicated R&D Center business is due in part to the expansion of the Bristol Myers Squibb R&D center.
Revenue performance in the first quarter was also boosted by the manufacturing of remdesivir to fulfil high demand for the drug from Indian healthcare providers.
During the quarter, the biologics business signed a five-year agreement with IAVI, a USA-based, non-profit, scientific research organization, to develop and manufacture three recombinant, monoclonal antibodies (mAbs) for HIV. The mAbs will be used for phase I and II human clinical studies. Under the agreement, Syngene will provide an integrated solution including clone selection, analytical methods development, manufacturing process development, scale-up and cGMP manufacturing of drug substance, viral clearance studies, cGMP manufacturing of drug product and stability studies.
Syngene scientists continued to work on the coronavirus and have generated several variants of the SARS-CoV2 spike S1 protein including the Alpha and Beta variants. These variants are used to determine the efficacy of different vaccines to cross-protect people from these strains.
Board updates
Dr Kush Parmar was appointed as a non-executive independent director on the Board effective June 22, 2021 until the conclusion of the Annual General Meeting (AGM) of the Company to be held in 2024, subject to the approval of the shareholders at the AGM scheduled on July 21, 2021. Dr Parmar holds a BA in molecular biology and medieval studies from Princeton University, a Ph.D. in experimental pathology from Harvard University and an MD from Harvard Medical School. Currently, he is a managing partner at 5AM Ventures, a life sciences venture capital firm headquartered in San Francisco. Dr Parmar serves on the advisory boards of Harvard Medical School, Penn Medicine, Princeton University’s Department of Molecular Biology and the Grace Science Foundation. At Princeton University, Dr Parmar worked on developmental genetics with Nobel Laureate Eric F. Wieschaus. A founding member of the COVID-19 R&D alliance, Dr Parmar also serves on the Boards of Akouos, Entrada, Homology, Rallybio and Vor Biopharma.
John Shaw retires from the Board after 21 years
John Shaw, non-independent director on the Board, will retire due to health reasons, at the end of the 28th Annual General Meeting of the Company, scheduled on 21st July, 2021. John was appointed to the Board in 2000 and has made significant contributions in helping the Company cross many significant milestones. His strong management and financial experience helped Syngene establish the strong corporate governance that the Company is recognized for. The Board places on record its deep appreciation and gratitude for the many years of his stewardship and guidance.
Earnings call
Syngene will host an investor call at 02.00 pm IST on July 21, 2021 where the senior management will discuss the Company’s performance and answer questions from participants. Please dial the numbers provided below ten minutes ahead of the scheduled start time to participate in this conference call. The dial-in number for this call is +91 22 6280 1279/ +91 22 7115 8180. Other toll numbers are listed in the conference call invitation which is posted on the Company website www.syngeneintl.com. The operator will provide instructions on asking questions before the start of the call. A replay of this call will also be available until July 28, 2021 on +91 22 71945757/ +91 22 66635757, Playback ID: 04006. We will aim to post the transcript of the conference call on the Company website within seven working days of the investor conference call.